 Theory of Stuttering – Blog
Theory of Stuttering – Blog
In July 2019, I posted a figure (Fig. 21) in which two components of a stuttering event are symbolized: an ‘impelling’ component (depicted in red) representing the person’s will to speak, and an ‘inhibiting’ component (depicted in blue) that interrupts the flow of speech against the person’s will. The basal ganglia are part of the first component, the cerebellum is part of the second one in that figure. This view was influenced by the ‘dual premotor model’ proposed by Goldberg (1985) and applied to stuttering by Alm (2006, 2007). The model reflects the traditional assumption that basal ganglia and cerebellum are anatomically separate subcortical systems that perform unique functional operations and interact only at the level of the cerebral cortex.
This view is obsolete; studies with animals and humans found that cerebellum and basal ganglia interact at the subcortical level and form an integral network (Bostan & Strick, 2018; Wang et al., 2020). Thus, I had to once more consider the role of basal ganglia and cerebellum in stuttering, and in this post I will present what came out. I still maintain the view that stuttering is caused by invalid error signals in the monitoring system – ‘invalid’ in the sense that no speech error happened – and that the cerebellum is critically involved in the generation of these error signals (see Section 2.2 in the main text). However, the role of the basal ganglia (BG) is more complicated than I assumed earlier: They are not a combatant in the struggle between the two components of stuttering, they are the battlefield.
For a long time, I believed I would never understand the intricate BG mechanism (and I think it is not yet completely understood by the experts), but I got that, in terms of motor control, there are two main pathways through the BG: a so-called direct pathway that facilitates the execution of a planned voluntary motor action, and a so-called indirect pathway that inhibits a motor action (details below). These two pathways operate like two reins, one for Go and one for NoGo. The prevalence of the one or the other pathway determines what we do or don’t do. Both pathways are modulated by inputs from many cortical and subcortical areas, such that knowledge, experience, and inherent instincts can influence the decision in the BG. For instance, the direct pathway gets support by the nucleus accumbens whose activity is associated with the expectation of reward, whereas the indirect pathway is supported by the amygdala whose activity is associated with fear.
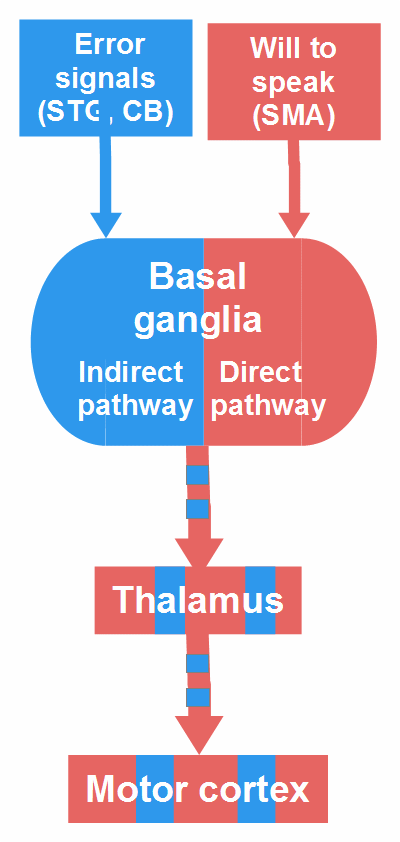 Figure 18: Two components of stuttering (update). STG = superior temporal gyrus, CB = cerebellum, SMA = supplementary motor area.
Figure 18: Two components of stuttering (update). STG = superior temporal gyrus, CB = cerebellum, SMA = supplementary motor area.
This figure is an update of the bottom part of Figure 17. I retain the symbolic colors: red for the impelling and blue for the inhibiting component. As mentioned above, the BG are part of both components. In fluent speech, the direct pathway facilitates the execution of selected speech motor programs. A stutter occurs when, by cerebellar input, the indirect pathway becomes transiently dominant and inhibits the execution of the selected or initiated speech motor program, whereas the speaker tries to continue. This attempt (= input from the frontal cortex) activates the direct pathway, and this activation contributes to overt stuttering symptoms: They would not occur, if the speaker, at the moment of inhibition, could give up the will to speak.
As mentioned, Figure 18 updates only the bottom part of Figure 17; regarding the upper part, I still think that error signals in the monitoring system (possibly a loop connecting superior temporal cortex, cerebellum, and thalamus) are caused by poor processing of auditory feedback as a result of a misallocation of attention during speech. An error signal at speech onset may occur when a speech motor program is initiated before the entire speech network, including attention and auditory system, is sufficiently prepared – or specifically, when speaking starts without any expectation of auditory input. The start may then be regarded as premature by the monitoring system and inhibited by the BG.
The rest of this blog post has been included in the main text, see Section 4.4.