 A Theory of Stuttering
A Theory of Stuttering
Up to now, there is no empirical evidence of the invalid error signals postulated to cause stuttering. However, a part of the brain involved in the detection and repair of motor errors on the basis of sensory feedback is the cerebellum. Zheng et al. (2013) identified a neural network that seems to encode an ‘error signal’ in response to distorted auditory feedback during articulation. The network includes right angular gyrus, right supplementary motor area, and bilateral cerebellum (read more about further studies).
With that said, the following findings concerning the cerebellar activation in stuttering are interesting: Several brain imaging studies including two meta-analyses (Brown et al., 2005; Budde et al., 2014) revealed that the cerebellum plays an important role in stuttering: During speech, it was found to be overactive in stutterers, compared to controls, and its activation was positively correlated with stuttering severity (Fox et al., 2000; Ingham et al., 2004).
More recently, Yang et al. (2016) found the resting state functional connectivity within cerebellar circuits to be correlated with the severity of stuttering. Furthermore, Kell et al. (2018) reported that, in individuals who spontaneously recovered from stuttering, the activity in the superior cerebellum together with the left prefrontal cortex (BA47/12) appeared uncoupled from the rest of the speech production network.
On the other hand, an impairment of cerebellar function can cause stuttering to disappear. Bakheit (2011) reported the case of a 54-year-old man who lost his lifelong stuttering after an ischemic infarct on the left side of the brainstem and in both hemispheres of the cerebellum. Two earlier cases had been reported by Miller (1985): severe stuttering disappeared with progressive multiple sclerosis and associated bilateral cerebellar dysfunction. A further case is that of Martina P. in 2012 in Germany; a scientific report is in progress but not yet published (read more).
Further evidence for an important role of the cerebellum in stuttering came from Wymbs et al. (2013). They used event-related fMRI to identify individual differences in the brain activation patterns of four stutterers during stuttered and fluent word production. They found that many brain regions were overly active during stuttered speech; however, the across-subject agreement for the activated regions was minimal. The only region that was overactivated during stuttered speech in all the four participants was the left cerebellum, lobule IV (see Table 2 in the paper).
A stuttering event consists of two components that work against each other: an ‘impelling’ component (depicted in red) that represents the person’s will to speak, and an ‘inhibiting’ component (depicted in blue) that interrupts the flow of speech against the person’s will.
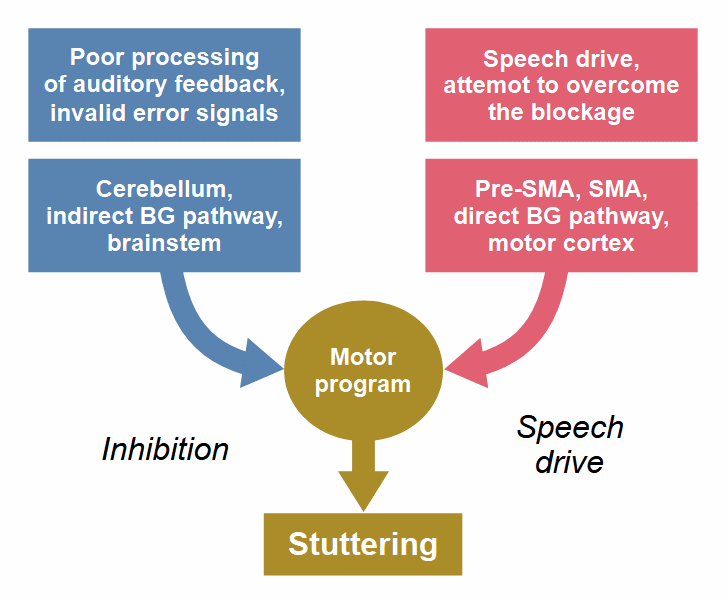 Figure 6: The two components of a stuttering event.
Figure 6: The two components of a stuttering event.
In an earlier version of this figure (see here), the basal ganglia were part of the right component, and the cerebellum was part of the left one, based on the ‘dual premotor model’ (Goldberg, 1985), which was applied to stuttering by Alm (2006). This model reflects the traditional assumption that basal ganglia and cerebellum are functionally separate and interact only at the level of the cerebral cortex. This view is obsolete. Studies with animals and humans found that cerebellum and basal ganglia interact at the subcortical level and form an integral network (Bostan & Strick, 2018; Wang et al., 2020). In the above figure, the cerebellum is still part of the left component only; it is the source of the invalid error signals that cause the inhibition of speech. The basal ganglia contribute to both components; they are not a combatant in the struggle between the two components of stuttering, but rather the battlefield.
There are two main pathways (ways of transmitting excitation) through the basal ganglia, a so-called direct pathway that facilitates the execution of a voluntary motor action, and a so-called indirect pathway that inhibits a motor action. These two pathways operate like two reins, one for Go and one for NoGo. The prevalence of the one or the other determines what we do or don’t do. Both pathways are modulated by inputs from many cortical and subcortical areas, such that our knowledge, experience, and inherent instincts can influence the decision made in the basal ganglia. For instance, the direct pathway gets support from the nucleus accumbens, whose activity is associated with the expectation of reward. In contrast, the indirect pathway gets support from the amygdala, whose activity is associated with fear, and from the cerebellum when an error in a motor sequence is detected.
The interaction between cerebellum and basal ganglia in stuttering, as I think it is likely, is described in detail and with figures in Section 4.4.
Martina&mbsp;P. had stuttered since the age of three. At age 52, she was diagnosed with a benign brain tumor left near the cerebellum. The tumor pressed the optic, the acoustic, and the vestibular nerves and had already grown right up close to the brainstem. In June 2012, the tumor was extirpated. Unfortunately, a brain bleeding occurred, and an emergency operation had to be done, because of which the patient was in a coma for some weeks. By the tumor or as a result of the complication, the cerebellum was damaged. Martina reported: “When I woke up from the coma, something was altered in my speaking. […] Something was missing—the stutter. […] Stuttering remained absent when speaking. I write ‘when speaking’ because it was still present in my thoughts; that is, I was waiting for the stutter at every word. But nothing came. Stuttering was […] so strongly linked to my emotions that, initially, it was hard for me to ‘accept’ the missing stutter.”
Martina has not stuttered since the OP until that day (May 2016). The weakness of the cerebellar function manifests in a motor impairment; she needs to use a wheeled walker and has not yet been able to type with ten fingers, which was perfect before the surgery. In the initial period after the OP, she also had difficulty speaking distinctly, which has, however, much improved meanwhile (sources: Der Kieselstein, magazine of the German stuttering association BVSS, issue 3, 2013, p. 46, and phone conversations with Martina in January 2015 and May 2016).
Martin Sommer, who is investigating this case at the Göttingen University, reports about it in the video (in German) from 35.23 to 39.50. Click on the video to watch from the start; click here to watch (on YouTube) from 35.23, where the part about the cerebellum begins. And here is the beginning of my translation:
“The loop that processes external signals does not run via the basal ganglia, but via the cerebellum […]. Thus, there is an additional preparation of voluntary movements sent via the cerebellum, which also finely adjusts movement. And here, this is quite interesting—originally, we did not think that this had influence, but in the self-help scene, we found a case repor.: A 52-year-old woman who had stuttered since childhood […]. Only because of a meningioma—an acoustic neuroma, sorry—and this was operated on, and the lady had bad luck. She got a cerebellar lesion left as a consequence of the neuroma surgery, that is, it had worked badly. Now, it is so that she has no longer stuttered since then...” (read the complete translation and my commetns here)
(return)
In a hand movement task, Blakemore, Frith, and Wolpert (2001) obtained results suggesting that the cerebellum is involved in signaling the sensory discrepancy between the predicted and the actual sensory consequences of movements. In a PET study of motor learning, van Mier and Petersen (2002) found an area in the left lateral cerebellum that showed practice-related decreases in activation, which were most likely related to a decrease in errors; in two of their experiments, a highly significant correlation was found between the decrease in errors and the decrease in left cerebellar activation.
Grafton et al. (2008) investigated the neural correlates of visuomotor tracking using fMRI and found that the activity in the cerebellum was correlated with the magnitude of tracking errors and motor corrections. Correlations between cerebellar activity and perceived errors were found in a reaching task (Diedrichsen et al., 2005) and in other visuomotor tasks (Miall, Imamizu, & Miyauchi, 2000; Miall, Reckess, & Imamizu, 2001; Miall & Jenkinson, 2005; Ramnani et al., 2000).
Seidler, Noll, and Thiers (2004) and Ogawa, Inui, and Sugio (2006) investigated feedback control and found the cerebellum to be more active under feedback control conditions in both studies, supporting a role for the cerebellum in feedback processes of motor control (see also Seidler et al., 2014, for an overview).
(return)